Sticky and Slimy: Nature’s Inspiring creations
October 6, 2011 in Fun Facts
The sticky and slimy properties of molecules are involved in many natural phenomenons.
Animals and plants, both can secrete viscous substances known as mucus. Mucus may act as a glue and a lubricant at the same time! Human and animal mucus is usually a “glycoprotein,” in other words a protein, such as the ones you eat, which is attached to complex carbohydrates (sugars and starches) often called ‘mucopolysaccharides’. Such mucus coats and protects different body parts such as the lungs and stomach. Snails, slugs, hagfish and some other creatures also produce external mucus. Such mucus has a protective function against infectious agents, toxins and predators. Mucus can also facilitate movement and communication.
Here are some examples of what we can find in nature!
Snail and slug mucus is called ‘pedal mucus’, because it is released from their foot and when mixed with water forms a thick jelly like material we call slime. Actually slime is a lot like the saliva in your mouth, containing mostly water (90%), some salts, and about 10% mucus. Snail and slug mucus is a little different from human mucus, because it is rich in complex sugars with a small amount of protein. By varying the amount of protein, slime can serve both as glue and a lubricant, preventing snails and slugs from drying out, absorbing and holding water.
A sweet and sticky deadly plant – the Sundew!
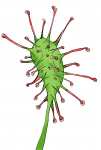 Sundews (Drosera) are “carnivorous plants”, which lure, capture, and digest insects. Sundews have tentacles. In the tentacles are glands, which secrete sweet sticky nectar to attract and trap insects, as well as digestive molecules called enzymes that break down the bug’s body. Attracted by the sweet secretions, insects become entrapped by this glue which prevents their escape. Within 15-30 minutes, the insect prey dies from exhaustion or by asphyxiation as the nectar envelops them. Plant enzymes dissolve the insect, making a nutritious insect soup, which is absorbed in the leaf surface and used to grow.
Sundews (Drosera) are “carnivorous plants”, which lure, capture, and digest insects. Sundews have tentacles. In the tentacles are glands, which secrete sweet sticky nectar to attract and trap insects, as well as digestive molecules called enzymes that break down the bug’s body. Attracted by the sweet secretions, insects become entrapped by this glue which prevents their escape. Within 15-30 minutes, the insect prey dies from exhaustion or by asphyxiation as the nectar envelops them. Plant enzymes dissolve the insect, making a nutritious insect soup, which is absorbed in the leaf surface and used to grow.
The magic of Spider webs
Spider web is made from spider silk. Special glands in the spider’s body make spider silk, which is lightweight, flexible, and pound for pound three times stronger than steel! The incredible properties of spider silk are due to its unique molecular structure. Spider silk is a protein composed of long chains of amino acids, which form crystals having great stiffness, strength and toughness. This crystalline component is mixed with a rubbery component composed of amorphous networks of amino acid residues, which permits extensibility (stretching). The interplay between the hard crystalline segments, and the elastic regions allow spider silk to stretch and still have high tensile strength, such that spider webs do not get damaged from wind or when catching bugs. Other compounds are used to enhance the fiber’s properties including moisturizers to keep the thread moist, and acids to protect it from fungi and bacteria that would otherwise digest the protein.
Mussel Power
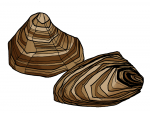 To avoid being washed away by waves, marine organisms secrete remarkable adhesive materials to stick to surfaces. Mussels, for example, produce sticky proteins, which are secreted as fluids that cross-link and harden to form a solid adhesive plaque, for attachment to different surfaces like metal, wood and stone even under water. One of the unique structural features of mussel adhesive proteins is the presence of L-DOPA, an amino acid that is believed to possess both adhesive and cross-linking properties. Chemists have reproduced such proteins in pursuit of synthetic glues.
To avoid being washed away by waves, marine organisms secrete remarkable adhesive materials to stick to surfaces. Mussels, for example, produce sticky proteins, which are secreted as fluids that cross-link and harden to form a solid adhesive plaque, for attachment to different surfaces like metal, wood and stone even under water. One of the unique structural features of mussel adhesive proteins is the presence of L-DOPA, an amino acid that is believed to possess both adhesive and cross-linking properties. Chemists have reproduced such proteins in pursuit of synthetic glues.
The adhesive secret of Geckos!
Geckos are lizards, found in warm climates throughout the world. Geckos are unique, because of their specialized toe pads that enable them to climb smooth and vertical surfaces, and even cross indoor ceilings with ease. Geckos adhere to most surfaces without the use of liquids nor surface tension. They have feet coated with bristle-like structures called setae, which hold geckos to surfaces by weak chemical forces called “Van der Waals interactions”. Every square millimeter of a gecko’s footpad contains about 14,000 hair-like setae. Each seta has a diameter of 5 micrometers. Human hair varies from 18 to 180 micrometers, so a human hair could hold between 3 and 36 setae. By using numerous weak chemical forces combined, geckos can adhere to almost any kind of surface.









